
Chances are good that if you’ve done any 3D printing, it was of the standard fused deposition modeling variety. FDM is pretty simple stuff — get a bit of plastic filament hot enough, squeeze the molten goo out of a fine nozzle, control the position of the nozzle more or less precisely in three dimensions, and repeat for hours on end until your print is done. To the outsider it looks like magic, but to us it’s just another Saturday afternoon.
Resin printing is another thing altogether, and a lot closer to magic for most of us. The current crop of stereolithography printers just have a high-resolution LCD display between a UV light source and a build tank with a transparent bottom. Prints are built up layer by layer by flashing UV light patterns into the tank as a build plate slowly lifts it up from the resin, like some creature emerging from the primordial goo.
Of course it’s all just science, but if there is any magic in SLA printing, surely it’s in the resins used for it. Their nondescript brown plastic bottles and information-poor labels give little clue as to their ingredients, although their hydrocarbon reek and viscous, sticky texture are pretty good clues. Let’s take a look inside the resin bottle and find out what it is that makes the magic of SLA happen.
The Basics
A good basis for understanding the chemical processes behind stereolithography resins is the polymerization of methylmethacrylate (MMA) into polymethylmethacrylate, also known as PMMA or simply acrylic. While formulations for SLA resins vary, many of them are based on acrylates, so the chemistry here is directly applicable to a lot of resins, as are the general principles.
The polymerization of methylmethacrylate is what’s known as a free-radical reaction. It works because MMA has a double bond between two of its carbon atoms, as well as a nearby ester group — the group with two oxygens, one of them in a double bond. The electronic structure of these two groups makes the double-bonded carbon susceptible to reduction, which is the gain of an electron.
Under normal circumstances, MMA monomers don’t react with each other because there are no free electrons floating around to reduce the carbon-carbon double bond. To get MMA to polymerize, an initiator — in this case, benzoyl peroxide — needs to be added to the mix. An initiator is a chemical compound that provides unpaired electrons, or free radicals. Once radicals are present, they bind to the carbon by reducing the double bond. The product of this first reaction will have its own unpaired electron, which can then go on and reduce the double bond in another MMA monomer, and so on. The production of a free radical product after initiation is the key to free-radical polymerization.
So it stands to reason that a bottle of SLA resin will contain monomers of MMA and an initiator of some sort. But what keeps the monomers from just polymerizing in the bottle? If the initiator was something like the benzoyl peroxide used in the example above, that’s exactly what would happen. So to be useful for SLA work, the resin mix has to contain an initiator that can remain inert in the mix until it’s needed.
Kicking Things Off
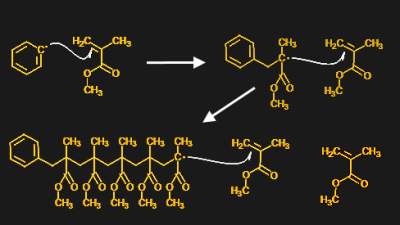
This is where photoinitiators come into play. Where an initiator like benzoyl peroxide will readily decompose into free radicals with the application of a little heat, photoinitiators need a little more coaxing. Hundreds of different photoinitiators have been developed by chemical companies over the years, each tailored to the specific set of monomers to be polymerized as well as to industrial needs, such as the efficiency of free radical formation, toxicity, and even odors imparted on the finished product. But they all share the common trait of being inactive until they are exposed to light of the correct wavelength.
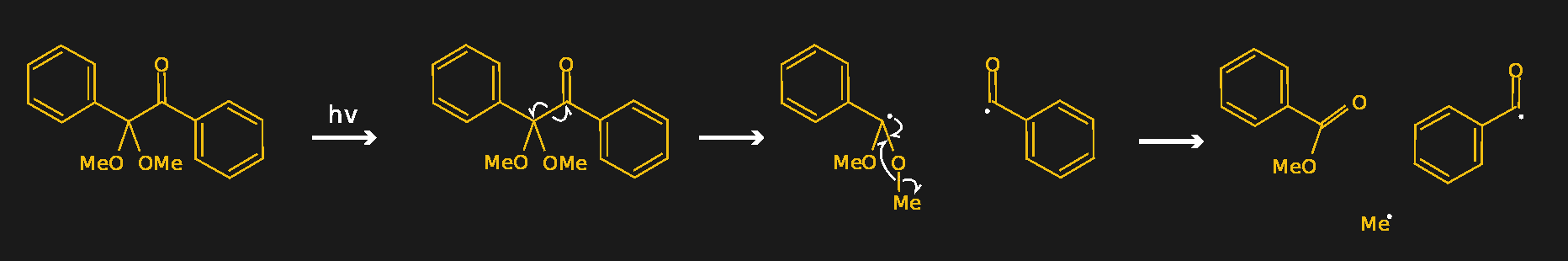
A good example of a photoinitiator is 2,2-dimethoxy-2-phenylacetophenone, mercifully abbreviated to DMPA and sold under the trade name IRGACURE 651 by Ciba. The compound has two benzene rings joined by a two-carbon chain. One of the carbons in the linker section is double-bonded to oxygen, forming a ketone functional group. When photons of the right wavelength — DMPA has absorption peaks are 250 nm and 340 nm — hit the ketone group, it becomes excited to the point where an electron is knocked off. Through a series of intermediate steps in which the spare electron is shuffled around to different atoms, the linker section of the molecule breaks in a process called α-cleavage. This leaves behind a stable species — methylbenzoate — plus two free radicals that can initiate polymerization.
Tapping the Brakes
The mechanism of photopolymerization begs a question: how does the UV light in an SLA printer not just polymerize the whole tank of resin at once? It seems like that would be a problem, since polymerization is basically a chain reaction once initiated. But there are practical limits to the reaction, for both chemical and physical reasons.
Chemically, the amount of initiator in the resin is typically pretty low — just a few percent of the mix. So there aren’t many places to start the polymerization reaction. Polymerization reactions also tend to undergo chain termination spontaneously, either by having two growing radical chains bind together, or by reduction of a radical chain by contaminants such as oxygen. Some resins even have specific inhibitor compounds added to limit the speed of polymerization. Either way, spontaneous termination keeps the tanks from becoming a solid brick of plastic.
There are also physical reasons for photopolymerization not running wild through the build tank. The UV light coming from the LCD display at the bottom of the tank isn’t particularly strong, and tends to get absorbed by the resin before traveling very far. This is why SLA resins tend not to be heavily pigmented, and why any pigments that are added to the resin have to be carefully selected to not absorb UV light. It’s also why SLA prints need an additional cleaning and curing step after printing; the polymerization that occurs in the tank is incomplete, with unreacted resin remaining inside the print. Bathing the print in high-intensity UV light completes the process and hardens the print.
Filling Up on Soy
Between initiators, monomers, pigments, and possibly inhibitors, SLA resins already seem like a witch’s brew of chemicals. But we’re not done yet. Resins rarely just use monomers, instead using a special blend of monomers and oligomers — short chains of pre-polymerized monomers. Adding oligomers into the resin tends to speed up polymerization by giving the growing chains a head start. It also tends to increase the viscosity of the resin, so that it’s not runny and doesn’t slosh around in the build tank and get bubbles.
Another common addition to SLA resins is a cross-linker. Cross-linkers are compounds that can form connections between two or more growing polymer chains. Cross-linking tends to make the polymer chain into more of a matrix structure, lending strength and rigidity to the final product. Cross-linking can also change the properties of the material, and even allows for copolymerization of different types of monomers, like adding urethane to acrylates to add toughness and flexibility.
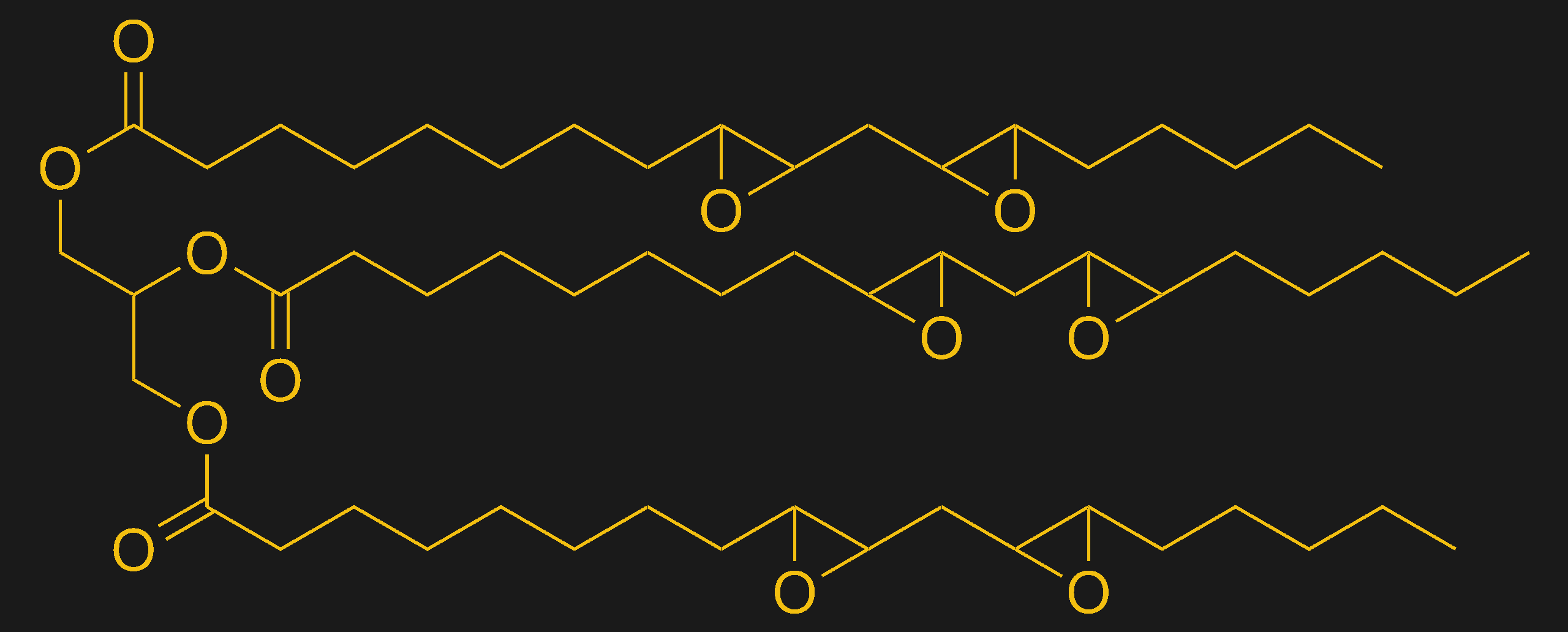
Some SLA resins also contain filler materials. Fillers are pretty common in plastics — a lot of Schedule 40 PVC pipe contains powdered limestone, for instance. In SLA resins, fillers are added to bulk up the plastic by filling the spaces between the cross-linked strands of polymers. A lot of new “eco-friendly” SLA resins are coming to claiming to be made from soybeans, and while that’s true — at least for some resins — there’s still a lot of material in the resin that’s clearly not from soybeans. And the soybean oil that’s in there is really just a filler — without the acrylate monomers and crosslinkers listed or the photoinitiator, the resin would be pretty useless.
Not Just for Printing
While we’ve concentrated mainly on SLA printing resins here, that’s far from the only application for photopolymers. If you’ve had a tooth filled any time in the last three decades or so, chances are good that your dentist used a photopolymer containing methacrylate monomers and cured with a fiber optic wand that emits UV light. Printed circuit board manufacturers make wide use of photopolymers, both in the photoresist coatings that are used to etch the boards, and in the solder mask that’s applied to the board. Photopolymers are also used for masking during the photolithographic processes involved in manufacturing integrated circuits.
0 Commentaires