Plastics, by and large, are well-understood materials. Not as strong as most metals, but often much lighter, these man-made polymers have found innumerable applications that have revolutionized the way we live. The properties of plastics have been improved in many ways over the years, with composite materials like fiberglass and carbon fiber proving to have strength and lightness far beyond the simple properties of basic polymers alone.
However, a group of engineers at MIT have been working on a revolutionary type of polymer that promises greater strength then ever before while remaining remarkably light weight. It’s all down to the material’s two-dimensional molecular structure, something once thought to be prohibitively difficult in the world of polymer science.
2D Is Better Than 1D
Typically, polymers assemble themselves into long one-dimensional chains, where multiple copies of the same molecular subunit, or monomer, links into a chain many times in what is often referred to as a macromolecule. These long molecules tangle together with themselves and each other in bulk, making up the polymer materials we know and love.
However, the manner in which monomers usually chain together has typically prevented any attempts to produce a polymer structure in two dimensions. If just one monomer attaches to another at the wrong rotation, further monomers will link onto it as well, creating a messy 3D structure instead of a neat and tidy 2D sheet.
With some careful synthesis, it turns out that a two-dimensional molecular polymer structure is indeed possible. As per the research paper published in Nature in February this year, this feat was achieved through the use of melamine molecules as the monomer unit. The working theory is that the use of amide-aromatic interactions in the synthesis steps inhibited the melamine molecules from rotating out-of-plane during the linking phase.
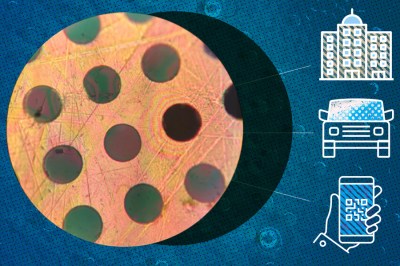
The material self-assembles into 2D sheets in solution, allowing for the creation of thin films of great strength. This structure also has the unique ability of being impermeable to gas molecules. The monomers lock together so closely that there’s simply no way for them to get through.
The resulting material is remarkable in its properties; the two-dimensional polymer was tested to have an astonishing yield strength of 976 MPa, almost four times that of structural steel, despite having a far lower density of just 1/6th as much. Meanwhile, the elastic moduli was measured to be around 30 to 90 GPa, significantly higher than traditional plastics which typically range from 3-5 GPa. This means the material is far stiffer and deforms less in the elastic regime compared to plastics like polycarbonate and nylon. This figure is far closer to that of metals like aluminium, which has an elastic modulus of 69 GPa.
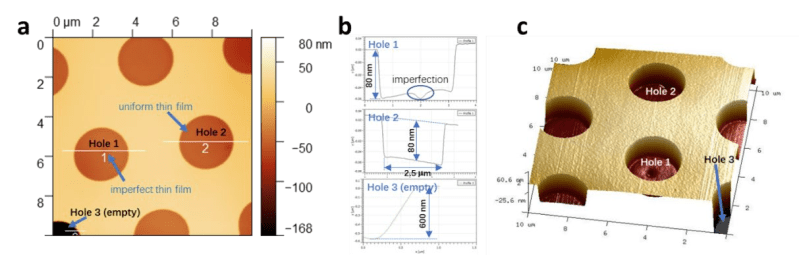
Of course, thus far, only tiny quantities of the 2D polymer have been created in the lab. Testing the material’s mechanical properties had to be done on the nanoscale, using a process called AFM nanoindentation. It allows microscopic samples to be tested using a hard indentation tip on an atomic force microscope to measure the material’s properties.
Importantly, the polymer as synthesized is mechanically and chemically stable. The paper’s authors suggest it has great potential for use in composite materials as well as for use as a lightweight but strong protective coating. It’s unclear at this stage how such a polymer could be produced at the macro scale, and it will likely be some time before this material is on sale in large sheets at your local plastic distributor. However, it shows that the world of science still has amazing secrets to be uncovered that could bring us new and wonderful materials beyond our wildest dreams!
0 Commentaires