
It’s often said that the best science experiments are the ones which do not require any special devices or ingredients, which makes the use of what naturally comes out of one’s body clearly one of the winners. It’s also the beginning of yet another [Hyperspace Pirate] chemistry video that’s both fascinating and unforgettable — this time introducing a considerable collection of urine, and the many uses of the urea in it, including its use for refrigeration.
As icky as this may sound, it doesn’t even rank in the top ten of quaint things people have historically done with urine, so extracting urea from it is rather benign. This is performed by adding sodium hydroxide to the starting component after heating, which creates gaseous ammonia (NH3) which was then condensed into its liquid (dissolved) form. In order to create the target compound – being ammonium nitrate – nitric acid (HNO3) had to be created first.
For this the older, but cheaper and easier Birkeland-Eyde process was used. This uses high-voltage electrical arcs to break down the nitrogen and oxygen in the air and cause the formation of nitric oxide (NO), that subsequently reacts with atmospheric oxygen to form nitrogen dioxide (NO2). Running the NO2 through water then creates the desired HNO3, which can be combined with the ammonia solution to create ammonium nitrate. The resulting solution was then evaporated into solid ammonium nitrate, to use it in an aluminium cooling cylinder, with freshly added water.
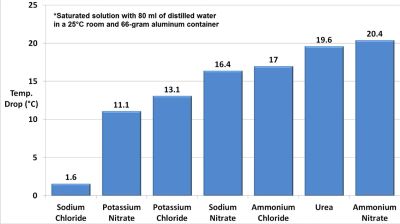
This is the simplest way to use the cooling effect of such solutions (pictured), but the benefit of ammonium nitrate over the original urea seems minimal. The low efficiency of this cooling approach means that the next use of urine will involve a much more efficient vapor-absorption cycle, which we’re sure everyone is squeezing their legs together for in anticipation.
We’ve been covering the refrigeration experiments [Hyperspace Pirate] has been conducting for some time now. If you’re into the science of making things cold check out how seashells can be turned into dry ice, or what goes into building a home cryocooler.
0 Commentaires